Automatic High-throughput Hybridoma Antibody Molecular Discovery Platform
Single B Cell Screening Platform
ADC Platform
Bispecific Antibody Platform
Clinical Trials
Papers





UC - Phase III clinical trial (Ranked 1st of clinical development in China and second globally)
CC & other - Phase II clinical trial (Ranked 1st of clinical development in the world)
Granted Fast Track Designation (FTD) & Orphan Drug Designation (ODD) by U.S. FDA
9MW2821 is the first therapeutic drug targeting Nectin-4 in the world to disclose clinical efficacy for indications of cervical cancer, esophageal carcinoma and triple-negative breast cancer.
9MW2821 is a novel Nectin-4 targeting ADC developed by ADC platform and automated high-throughput antibody discovery platform of Mabwell. It boasts the advantages of homogenous structure, high purity and being easy production. It has also demonstrated favorable druggability properties in binding affinity, endocytosis, preliminary in vivo and in vitro pharmacodynamic activities, drug metabolism properties and preliminary safety.
Clinical Study results (updated by June 5):
UC (37 patients evaluable for efficacy assessment):
ORR: 62.2%, DCR: 91.9%, mPFS: 8.8 months, mOS: 14.2 months.
CC (53 patients evaluable for efficacy assessment):
ORR: 35.8%, DCR: 81.1%, mPFS: 3.9 months, mOS not yet reached.
Nectin-4 tumor cell staining intensity 3+ (39 patients): ORR 43.6%.
EC (39 patients evaluable for efficacy assessment):
ORR: 23.1%, DCR: 69.2%, mPFS: 3.9 months, mOS: 8.2 months.
TNBC (20 patients with locally advanced or metastatic triple-negative breast cancer evaluable for efficacy assessment))
ORR: 50%, DCR: 80.0%, mPFS: 5.9 months, mOS not yet reached.


7MW3711 is a novel B7-H3 targeting ADC developed by Mabwell's IDDC™ platform for the treatment of advanced malignant solid tumors. It is composed of innovative antibody molecule, novel linker, and novel payload (TOP1i). When 7MW3711 enters human body, it specifically binds to antigens on the tumor cell membrane surface, be internalized and trafficked to the lysosome, release cytotoxic drug, and induce the apoptosis of tumor cells.
7MW3711 is pharmaceutical characterized as stable structure, homogeneous composition, high purity, and it is suitable for industrial scale-up. Compared with ADCs in the same class at home and abroad, 7MW3711 has shown better tumor killing effects in multiple animal tumor models. In the safety evaluation model of animals including cynomolgus monkeys, the on-target and off-target toxicities of 7MW3711 are effectively controlled, showing its good safety profile and pharmacokinetic properties. The above research results indicate that 7MW3711 has clinical differentiation characteristics and a promising future of clinical development.
9MW2921 is a novel trop-2 targeting ADC developed by Mabwell's IDDC™ platform for the treatment of advanced solid tumors. It is composed of innovative antibody molecule, novel linker and novel payload (TOP1i) . When 9MW2921 enters the body, it specifically binds to antigens on the cell membrane surface, be internalized and trafficked to the lysosome, release cytotoxic drug, and induce the apoptosis of tumor cells.
9MW2921 is pharmaceutical characterized as stable structure, homogeneous composition, high purity, and it is suitable for industrial scale-up. Compared with ADCs of the same class under development at home and abroad, 9MW2921 is significantly improved and optimized in endocytic activity, plasma stability, drug release characteristics, bystander killing effect, etc. In vivo pharmacodynamic studies demonstrated that 9MW2921 had a better tumor killing activity. In animal safety evaluation models including cynomolgus monkeys and rats, the on-target and off-target toxicities of 9MW2921 were effectively controlled, indicating that 9MW2921 has good safety profile and pharmacokinetic properties.
Denosumab is a fully human IgG2 monoclonal antibody. MAIWEIJIAN is the first denosumab biosimilar(120mg) approved in China for the treatment of giant cell tumor of the bone that is unresectable or where surgical resection may lead to severe functional impairment, including in adults and adolescents with mature skeletal development (defined as having at least one mature long bone and a weight of ≥ 45kg).
8MW0511 is recombinant (Yeast-secreted) Human Serum Albumin-human Granulocyte Colony-stimulating Factor Fusion Protein for Injection. Recombinant human granulocyte colony-stimulating factor (rhG-CSF) is engineered by human serum albumin fusion technology to increase the half-life of rhG-CSF in human and prolong the dosing cycle, and to slow release of rhG-CSF and continue to promote the development and release of neutrophils, thereby reducing the incidence, duration, and severity of chemotherapy-associated neutropenia.
New drug application of 8MW0511 has been accepted by NMPA for use in adult patients with non-myeloid malignant neoplasms to reduce the incidence of infections manifested by febrile neutropenia when receiving myelosuppressive anticancer drugs that are susceptible to febrile neutropenia.


6MW3211, developed based on Mabwell's Bispecific Antibody Platform, is designed to selectively bind to CD47 and PD-L1 on tumor cells to attenuate CD47-SIRPα signal and block the PD-1/PD-L1 checkpoint inhibition, thereby triggering stronger tumor cell phagocytosis and enhancing T cell activation. The IgG-like structure with a common light chain is the strategy to overcome pairing problem and simplify the production process.


First in China & top tier of global
MW3811 is an innovative humanized monoclonal antibody against IL-11, which can bind IL-11 through high affinity and effectively block the activation of IL-11 downstream signal pathway being developed for fibrosis and oncology.
Preclinical study showed that 9MW3811 bound to IL-11 with high affinity, effectively blocked the activation of IL-11 signaling pathway, specifically regulated the interaction of tumor cells with T cells, macrophages, and tumor-associated fibroblasts, and thus enhanced the release of inflammatory cytokines in the tumor microenvironment, and increased the infiltration of T cells. Combination anti-tumor efficacy with anti-PD1 antibodies has been observed in a variety of solid tumor models.
JUNMAIKANG(Adalimumab biosimliar) is a recombinant humanized anti-TNF-α monoclonal antibody injection jointly developed by Mabwell and Junshi Biosciences. The mechanism is that the antibody binds to TNF-α and reduces the TNF-α-activated immune response, thereby inhibiting the inflammatory response. JUNMAIKANG has been approved by NMPA for the treatment of rheumatoid arthritis, ankylosing spondylitis, psoriasis, crohn's disease, uveitis, polyarticular juvenile idiopathic arthritis, pediatric plaque psoriasis, and pediatric Crohn's disease.
First in China
9MW1911 is an innovative humanized monoclonal antibody independently developed by Mabwell, a domestic pharmaceutical company in China. The drug's antibody molecule is derived based on the B-lymphocyte screening platform, and the product is characterized by higher binding affinity and potent biological activity. Nonclinical studies have shown that the in vivo mechanism of action of this product in animals was definite and clear. After binding specifically to the target ST2, it blocks the activation of ST2-mediated signaling pathway induced by cytokine IL-33, hence inhibiting the inflammatory reactions and achieving therapeutic effects in multiple auto-immune diseases.



First in China & top tier of global
9MW3811 is an innovative humanized monoclonal antibody against IL-11, which can bind IL-11 through high affinity and effectively block the activation of IL-11 downstream signal pathway being developed for fibrosis and oncology.
In preclinical studies of fibrotic diseases, 9MW3811 significantly reduced the area of pulmonary fibrosis, reduced the content of lung collagen and improved lung function in mice with fibrosis, making it a promising therapeutic agent for idiopathic pulmonary fibrosis and other diseases.
MAILISHU (9MW0311) is the second approved denosumab biosimilar worldwide for the treatment of osteoporosis. Denosumab is a fully human IgG2 monoclonal antibody. It is used to treat bone loss (osteoporosis) in people who have a high risk of getting fractures by acting as a RANKL (receptor activator of Nf-kB ligand) inhibitor to prevent osteoclastic-medicated bone resorption.


First in China & top tier of global
MW3011 (R&D code in the US: MWTX-003/DISC-3405) is an anti-TMPRSS6 antibody developed by Innovation R&D Center of Mabwell in San Diego, U.S. With its target mainly expressed on the surfaces of liver cell membranes,
9MW3011 can upregulate the level of hepcidin expressed by hepatocytes through specific binding, inhibit the absorption and release of iron, and lower the serum iron level, thus regulating the iron homeostasis in vivo. The proposed indications of 9MW3011 include β-thalassemia and polycythemia vera. At present, there are no mature and effective macromolecular drug for relevant indications. FDA has granted Fast Track Designation (FTD) to 9MW3011.
Mabwell has granted DISC Medicine, INC. (NASDAQ:IRON) exclusive rights to develop and commercialize 9MW3011 in the United States, Europe, and other territories excluding Great China and Southeast Asia.
9MW0813 (aflibercept biosimilar) is an independently developed recombinant human VEGF receptor-antibody fusion protein. 9MW0813 is a fusion protein formed by recombining the extracellular region binding domains of VEGFR-1 and VEGFR-2 with the Fc segment of human immunoglobulin, which can bind to VEGF-A and PlGF, and has wide applications scope. It is expected to be complementary in the treatment of ocular diseases related to neovascularization.
9MW0211 is a recombinant anti-VEGF humanized monoclonal antibody obtained based on rabbit monoclonal antibody and humanization modification technology. It can specifically bind to VEGF-A, the most active protein in VEGF family, and block its binding to receptors on the surface of endothelial cells, reducing vascular permeability and blocking the generation and development of neovascularization, and reducing leakage caused by neovascularization, thus achieving the treatment of neovascularization-related eye diseases such as neovascular (wet) age-related macular degeneration.
Mabwell has built an automatic high-throughput hybridoma antibody molecular discovery platform, which is equipped with international advanced equipment, has an independently integrated workstation system, and is combined with a variety of animal immune technologies, efficient and stable hybridoma electrofusion technology, serum-free hybridoma suspension culture technology, real-world flow screening technology and many other underlying technologies. Meanwhile, in the antibody engineering transformation optimization system composed of computer-aided design and various display technologies, the platform adds physical and chemical stability indicators such as antibody expression characteristics, molecular binding epitope and hydrophobicity to ensure that the obtained innovative molecules meet the needs of industrialization. In addition, the platform also has a unique affinity mature transformation technology. On the basis of maintaining the activity of antibody molecules, it can greatly improve the binding affinity of antibody molecules, effectively improving the probability of druggability of innovative molecules.
 Advantages:
Advantages: 1) The target development scope is wider and the immune success rate is improved from the source.
2) Highly efficient, stable and reproducible hybridoma electrofusion technique, increasing the hybridoma screening abundance, and being conducive to obtaining candidate antibody molecules.
3) Workflow integrating manipulator and high-throughput antibody sorting equipment.
4) Serum free hybridoma suspension culture significantly accelerated the cloning and screening and reduce the incidence of false positive.
5) Antibody multi property evaluation system for cell stereoepitope level.
Mabwell’s high efficiency B lymphocyte screening platform is based on the direct separation of antigen-specific B lymphocytes from the spleen of immunized animals and human peripheral blood, and the antigen-specific B lymphocytes were enriched and the primary B lymphocytes were cloned and expanded by using the proprietary technology of efficient panning and clonal amplification. The use of efficient panning technology has achieved the screening of one hundred thousand B lymphocytes that can specifically bind to antigen from one hundred million B lymphocytes, the positive rate of secreted antibody binding to antigen is more than 90%, which significantly improves the discovery rate of high affinity antibody molecules, reduces the loss of positive B lymphocytes and improves the abundance of candidate antibodies in the process of panning. The high affinity antibody gene that is difficult to obtain by conventional cell fusion can be obtained by using the high-efficiency B lymphocyte screening technology, thus, better candidate antibody molecules are obtained, which enriches the technical means of new antibody molecule discovery.
 Advantages:
Advantages: 1) The positive rate of antigen-specific B lymphocytes was significantly increased.
2) Naturally stable antibody sequences can be obtained by screening.
3) The antibody molecular screening process has high fidelity, and the operating samples can be frozen for a long time.
4) Antibody molecular screening has strong pertinence and low research cost.
5) The technique is highly versatile and can achieve cross-species adaptation.
Mabwell’s ADC Platform is established based on two third-generation antibody coupled drug technologies, namely bridged fixed-point coupling technology and dispersed fixed-point coupling technology. Two different coupling technologies have submitted patent applications for connexon. The coupling process is reliable and the coupling product is more uniform and better than the ADC developed by other bridging fixed-point technologies. Compared with other types of antibody-drug conjugates, it has better pharmacokinetics, pharmacological and toxicological characteristics.
IDDC™ is a next generation ADC site-specific conjugate technology platform independently developed by Mabwell. It is composed of multiple systematized core patent technologies including site-specific conjugate process DARfinity™, special designed linker IDconnect™, novel payload Mtoxin™, and conditional release structure LysOnly™, which improves structural homogeneity, quality stability, pharmacodynamics and tolerability of the ADC products.
 Advantages:
Advantages: 1) Two different coupling technologies can develop ADC drugs for different types of high activity small molecule drugs.
2) The two different coupling techniques are applicable to the common antibody IgG1, and the natural antibody sequence can be used directly.
3) The conjugates have excellent uniformity, simplified process, easy quality control, and can significantly expand the therapeutic window in the process of use.
Mabwell's Bispecific Antibody Platform has three mature design schemes of Fc fusion protein like double antibodies in the form of common light chain, heterodimer structure and head tail structure, which can be optimized according to the characteristics of different double antibodies/proteins, The key common problems of engineering cell line screening, production process and quality control were solved, which laid a foundation for the comprehensive expansion of double antibody technology.
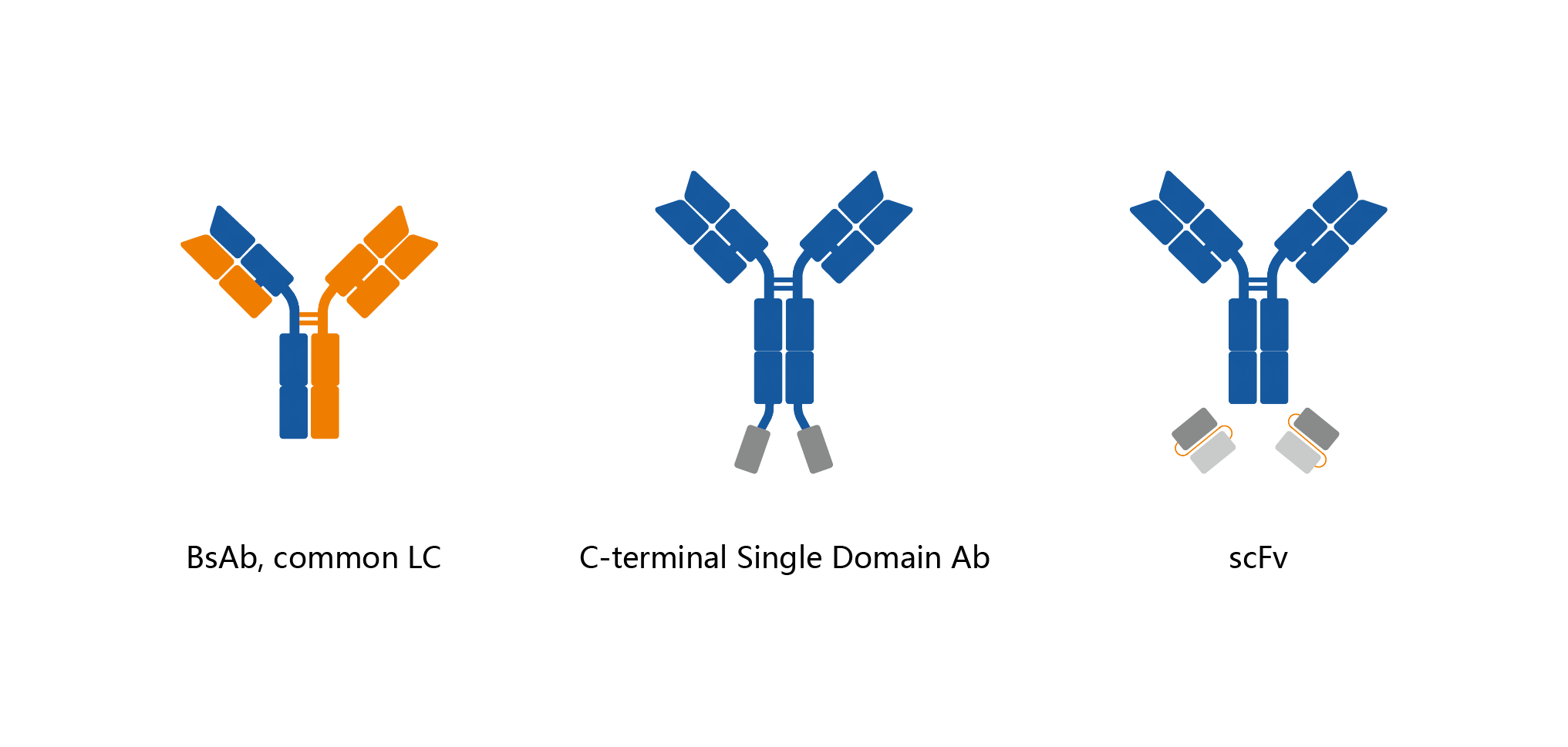 Advantages:
Advantages: 1) Differential design based on the molecular characteristics and functional requirements of the antibody reduces the difficulty of process development and quality control in the development stage and even the commercial production stage.
2) Take design as the source to solve the difficulties in process development, improve the stability of antibody molecules, improve the expression in the culture process.
3) Significantly reduce the production cost of bispecific/bifunctional antibodies, make the products more clinically accessible after commercialization.

Indications: treatment of adults and skeletally mature adolescents (defined as at least 1 mature long bone and weighing ≥ 45 kg) with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.

Indications: treatment of postmenopausal women with osteoporosis at high risk for fracture. In postmenopausal women with osteoporosis, MAILISHU reduces the incidence of vertebral, nonvertebral, and hip fractures.

Indications: rheumatoid arthritis, ankylosing spondylitis (AS), psoriasis, crohn’s disease, uveitis, polyarticular juvenile idiopathic arthritis (pJIA), pediatric plaque psoriasis, pediatric crohn’s disease